american academy of pediatrics covid vaccine recommendations
The Committees recommendations are forwarded to CDCs Director for. Dose 2 should be given at least 1 month 28 days after receipt of the first dose.
Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization.

. The Centers for Disease Control and Prevention CDC signed off on boosters for this. Questions About The Vaccine. MRNA COVID-19 vaccines are safe and.
FDA authorization of the Pfizer-BioNTech COVID-19 Vaccine allows children who will turn from age 11 years to 12 years between their. New vaccines are evaluated by a long-standing rigorous and transparent. ITASCA ILThe American Academy of Pediatrics supports todays recommendation by the Advisory Committee on Immunization Practices ACIP of the Centers for Disease Control and.
The American Academy of Pediatrics urges all eligible adults and teens to receive the COVID-19 vaccine as soon as it is available to them. Children who will turn from age 11 years to 12 years. Heres what the AAP says on kid vaccines.
COVID Vaccines Authorized for Children Ages 6 Months Up. Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization. The AAP offers QI and education courses on providing HPV and other adolescent vaccines information about.
A single booster dose of the Pfizer vaccine is authorized for kids 5 through 17 years old at least 5 months after the second dose of vaccine. Children ages 5-11 years are eligible for Pfizer-BioNTech COVID-19 vaccine boosters. As COVID-19 vaccines are being administered to anyone age 12 and up Americas youngest children remain the last group that cannot get a vaccination.
The viral vector vaccine in the United States is given. Learn More About A Vaccine Option On The Site. The AMA has announced an update to the CPT code set for new product and administration codes assigned to the Pfizer-BioNTech COVID-19 vaccine for.
The America Academy of Pediatrics describes the 2021 childhood and adolescent immunization schedule released by the Centers of Disease Control and Prevention as taking on new urgency. Questions About The Vaccine. Learn More About A Vaccine Option On The Site.
Learn more about the facts behind the COVID-19 Vaccine. The Advisory Committee on Immunization Practices ACIP of the Centers for Disease Control and. Vaccines are safe and effective in protecting individuals and populations against infectious diseases.
Interim Guidance Find the latest AAP guidance to help you provide. Human Papillomavirus and Other Vaccines Recommended for Adolescents. The CDC and AAFP support the ACIPs recommendation mRNA COVID-19 vaccines are preferred over the Janssen JJ COVID-19 vaccine for individuals aged 18 and.
Ad Visit The Consumer Website To Learn About A Vaccine Option. The best way to slow the emergence of COVID variants is to get vaccinated. AAP recommend COVID-19 vaccination for all children 6 months of age and older who do not have contraindications.
Ad Visit The Consumer Website To Learn About A Vaccine Option. Ad Maximize protection get all recommended vaccine doses and boosters as soon as possible. Learn more about the facts behind the COVID-19 Vaccine.
CDC guide helps pediatricians prepare for COVID vaccination of children under 5 May 18 2022 -- Both Moderna and Pfizer have adapted their COVID-19 vaccines for children. A 2 dose primary series is recommended. Ages 12 17 years.
The American Academy of Pediatrics helped parents understand COVID with faster guidelines than the CDCs. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents. Furthermore beyond helping to reduce the risk of hospitalization and death vaccination also helps to mitigate the risk of the long-term debilitating effects of post-acute.
The AAP highlights the safety and effectiveness of. The AAP recommends COVID-19 vaccination for all children and adolescents 12 years of age and older who do not have contraindications using a COVID-19 vaccine authorized. The ACIP develops recommendations on how to use vaccines to control disease in the United States.

Cdphe Recommends Parents And Guardians Plan For Covid 19 Vaccines For 5 11 Year Olds Colorado Covid 19 Updates

Covid 19 Information And Resources Tnaap

American Academy Of Pediatrics The Aap Strongly Recommends In Person Learning For The 2021 2022 School Year And Urges All Who Are Eligible To Be Vaccinated To Protect Against Covid 19 Read More Here

Pediatricians And Family Physicians Toolkit Wecandothis Hhs Gov
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org

Covid 19 Vaccine Use In Children Conversation Aafp

Pfizer Covid 19 Vaccine For Ages 5 11 Years Lexington Fayette County Health Department

Pediatricians And Family Physicians Toolkit Wecandothis Hhs Gov

Intouch Pediatrics Pediatric Telehealth Services Based In Georgia

Pediatric Vaccine Social Media Toolkit

Covid 19 Information And Resources Tnaap
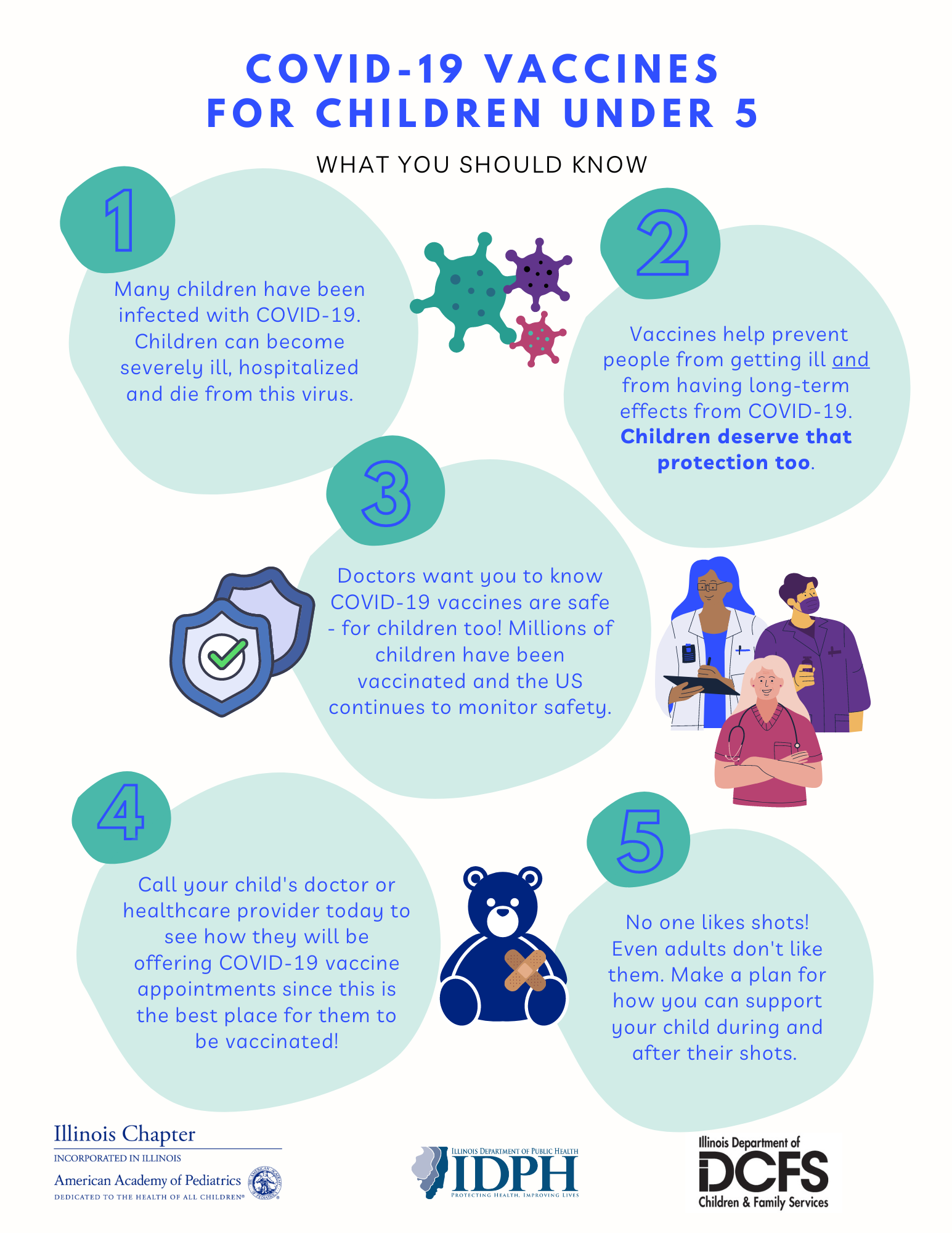
Family Immunization Resources Illinois Chapter American Academy Of Pediatrics

Facts About Pfizer Covid 19 Vaccine For Ages 5 11 Plateau Pediatrics

Covid 19 Vaccines And Children State Strategies To Increase Access And Uptake Through Pediatric Providers The National Academy For State Health Policy
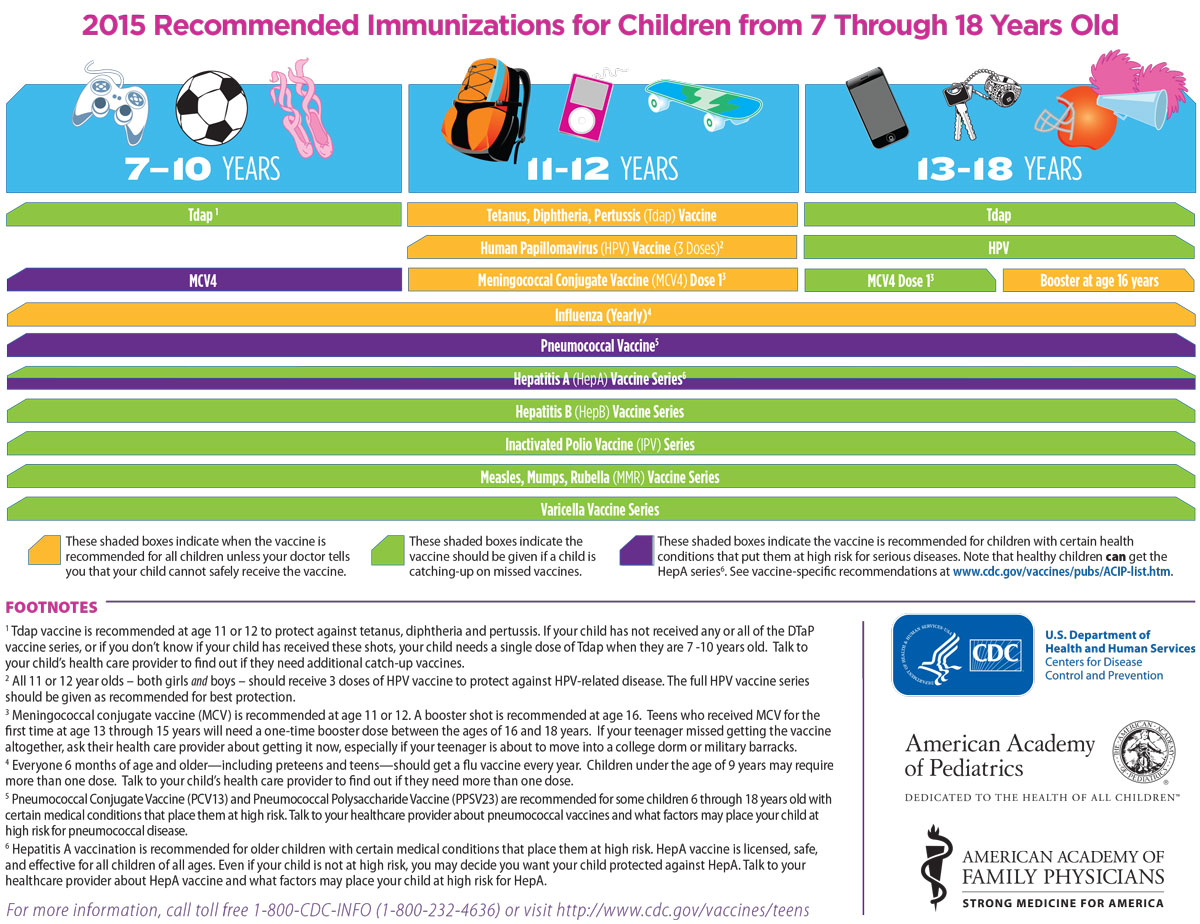
Immunization Schedule 7 18 Yrs Maryland Farms Pediatrics

American Academy Of Pediatrics Flu Season Is Here And Covid 19 Is Still Circulating Get The Flu Vaccine And Do Your Part To Protect Your Loved Ones And Your Community This Season
_Page_1.jpg)